Clinical Laboratory Management (C-CLM)

IFCC C-CLM Committee
Top L-R: Raja Elina, Member; Prasenjit Mitra, Member; Merve Sibel Gungoren, Member; Sanjaya Kumar Shah, Corr. Member; Praveen Sharma, C-CLM Chair
Down L-R: Mahesheema Ali, Member; Shruti Gupta, Corresponding member.
(Picture location: IFCC C-CLM Functional Meeting in IFCC WorldLab Congress 2022 Seoul (28 June 2022))
IFCC webinar on
“Risk Management in Clinical Laboratories”
is available on demand
This webinar provides an overview of risk management in healthcare focusing on clinical laboratories. The presentations provide an explanation of how Risk Management is done and highlight Risk Management frameworks like Failure Modes and Effects Analysis (FMEA), Root Cause Analysis (RCA), and others. The webinar contains three presentations of 20 min per speaker plus 20 min panel discussion.
___________________________________________________________________________________________
Clinical Laboratory Management (C-CLM)
Terms of Reference - Membership details - Roles and Responsibilities - Publications and Survey Reports
Meeting and Events - Clinical Laboratory Management Toolbox - Glossary of terms

Membership
| Name | Position | Country | Term | Time in Office |
| P. Sharma | Chair | IN | 2nd | 2023 01 - 2025 12 |
| M.S. Güngören | Member | TR | 2nd | 2023 02 - 2025 12 |
| R. Elina | Member | MY | 1st | 2021 03 - 2023 12 |
| M. Ali | Member | US | 1st | 2022 03 - 2024 12 |
| P. Mitra | Member | IN | 1st | 2022-03 - 2024 12 |
Mission and Purpose
The Committee on Clinical Laboratory Management (C-CLM) was established as an expert group focusing on and setting up activities in line with the common interests and needs of clinical laboratories particularly in developing countries. The primary goals of the C-CLM are:
• to provide education and training on good laboratory practice and structuring laboratory management in compliance with the globally recognized framework of quality system essentials;
• to help set standards/guidelines/requirements for implementing quality management that impact day-to-day work in the clinical or medical laboratories and, find solutions to conformity assessment issues at fulfilling their regulatory requirements;
• to promote good leadership and management practices in clinical laboratories and to assist with development of these skills among clinical laboratory professionals;
• to produce monographs and/or guides for those embarking on executing a quality management system and seeking accreditation.
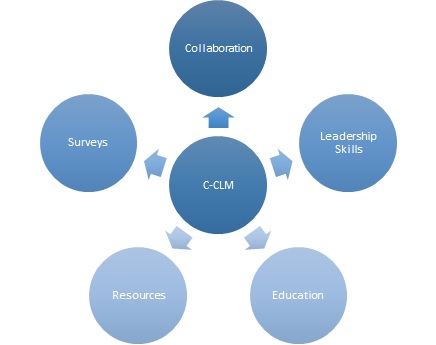
The C-CLM purpose will be accomplished through activities in five key areas:
1. Promoting Development of Strong Leadership and Good Management Skills among Laboratory Professionals
2. Producing Educational Materials
3. Providing Clinical laboratory Management Resources
4. Conducting Surveys to determine needs and demands
5. Collaborative projects with other IFCC Committees and Working Groups.
Target Groups
All laboratory professionals wishing to develop good clinical laboratory leadership and management skills. While focus will be on the needs of developing countries, developed materials will be applicable and useful to all levels of management and leadership experience.
Definition of Terms
By "clinical laboratory" we mean a facility for the examination of materials derived from the human body for the purpose of providing information for the diagnosis, prevention, or treatment of any disease or impairment of, or the assessment of the health of, human beings. These examinations also include procedures to determine, measure or otherwise describe the presence or absence of various substances or organisms in the body. Facilities restricted to collection and/or preparation of specimens; or only serving as a mailing service, but not performing testing; only performing POCT (point of care testing); or providing only direct-to-consumer testing (DTCT) are not considered clinical laboratories.
By "laboratory professional" we mean clinical laboratory specialists including laboratory directors, technical consultants, clinical consultants and clinical or medical laboratory science professionals, medical laboratory technologists, and medical laboratory technicians. The latter two professions are often called medical laboratorians and are directly involved in uncovering and providing laboratory information from laboratory analyses that assist physicians in patient diagnosis and treatment, as well as in disease monitoring or prevention (maintenance of health). Laboratory professionals use sophisticated biomedical instrumentation and technology, computers, and methods to perform laboratory testing on blood and body fluids, monitors testing quality, and consults with other members of the healthcare team.
Committee Chair
Prof. Praveen Sharma
MSc (Med),PhD (Med),FACBI, FAMS, FAACC
Head, Department of Biochemistry,
Dean ( Research),
Controller of Examinations,
All India Institute of Medical Sciences,
Jodhpur-342005 - India
Email: praveensharma55@gmail.com
sharmapr@aiimsjodhpur.edu.in

